
MULTI-CRITERIA OPTIMIZATION IN RAYSTATION
Treatment planning for radiation therapy inevitably involves compromises between dose to the tumor volume and sparing of healthy structures. These tradeoffs are conventionally handled using trial and error, where parameters such as objective function weights are adjusted and the treatment plan is reoptimized multiple times. Manual parameter tuning is inefficient, and the quality of the result dependent upon the experience and skill of the treatment planner. Multi-criteria optimization provides a more streamlined and intuitive workflow, where the clinical plan is selected by continuous navigation over the set of possible plans. It enables clinicians to make informed and structured decisions regarding how to best treat patients.
INTRODUCTION
The multi-criteria optimization (MCO) module allows for the exploration of treatment plans where no objective can be improved without worsening another. Treatment plans that satisfy this criterion are called Pareto optimal. The high-level workflow for MCO involves the formulation of an optimization problem, the calculation of Pareto optimal plans, plan exploration through navigation, and the selection of the preferred plan for treatment delivery. The MCO module has been developed in close collaboration with the Department of Radiation Oncology at Massachusetts General Hospital in Boston, MA, USA.
Treatment planning system RayStation\* supports MCO for intensity-modulated radiation therapy (IMRT) with the multileaf collimator (MLC) either in static (SMLC) or dynamic (DMLC) mode. Volumetric modulated arc therapy (VMAT), tomotherapy, and proton pencil-beam scanning are also supported. More detailed information about MCO for tomotherapy is available in a separate white paper [1].
OPTIMIZATION PROBLEM FORMULATION
Formulation of planning objectives is more straightforward in MCO than standard inverse planning. This is because preferences in terms of weights do not need to be articulated. In contrast to standard planning, objectives in MCO should reflect ideal criteria, such as a uniform dose at the prescription level for targets and no dose to organs at risk (OARs). Bare-minimum requirements, such as minimum acceptable target coverages and maximum acceptable doses to OARs, are entered as constraints in order to limit the Pareto optimal set to clinically relevant plans.
MCO may be combined with robust optimization using robust constraints, in order to safeguard against perturbations such as setup shifts, organ motion, and range shifts for ion beams. The lack of preference information makes MCO formulations ideal for creation of templates that can be reused for multiple patients.
PARETO PLAN GENERATION
In theory, the potential number of Pareto optimal plans is infinite. These plans are approximated by a user-selected number of Pareto plans, of which there are three types:
• Anchor plans that are generated by optimization with respect to a single objective, disregarding all others.
• A balance plan that gives equal emphasis to all objectives.
• Auxiliary plans that are constructed toward minimization of the overall approximation error of the current representation of the Pareto optimal set.
Auxiliary plans are generated toward minimization of the distance between an inner and outer approximation of the Pareto optimal set [2] if the number of objectives is less than ten. Otherwise, they are generated by optimization with respect to pairwise combinations of the most anti-correlated objectives. A feasibility check is performed at the start of the Pareto plan generation, which terminates with a summary of the violated constraints if the problem is infeasible.
Pareto plans for SMLC, DMLC, and VMAT can be generated using either fluence map optimization or direct machine parameter optimization (DMPO). The latter option makes the navigated dose distribution a more realistic representation of an achievable plan, but it also requires more time-consuming calculations.
Tomotherapy and pencil-beam scanning plans are always generated by DMPO. To enable the direct generation of a plan that closely resembles the navigated dose, the MLC leaf motions during DMPO for DMLC and VMAT are constrained to be unidirectional within start and end positions that are identical across all Pareto plans. The leaves alternate between left-to-right and right-to-left unidirectional motion over gantry angle intervals that have a length of at most 24° for VMAT.
Beam settings can be used to enforce common parameter limitations across all Pareto plans, for example jaw limits, bounds on delivery time or number of monitor units, and spacings for spots and energy layers of proton beams.
NAVIGATION
The navigation is controlled using slider bars associated with the objectives, see Figure 1. Slider movements are translated by a navigation algorithm to updates of coefficients that determine how the navigated dose is linearly interpolated from the Pareto plans. The sliders move in continuous fashion and all updates of the navigated dose distribution and associated dose–volume histogram (DVH), dose statistics, and clinical goals occur in real time. Movement of one slider in general causes all other sliders to change due to correlation between the objectives. Undesired slider movements can be prevented by clamps that enforce limits on the range of possible movements for the other sliders.
In addition to manual navigation, the module also supports automatic navigation toward fulfilment of a prioritized list of clinical goals. The automatic navigation is implemented by lexicographic optimization where the plan coefficients constitute optimization variables.
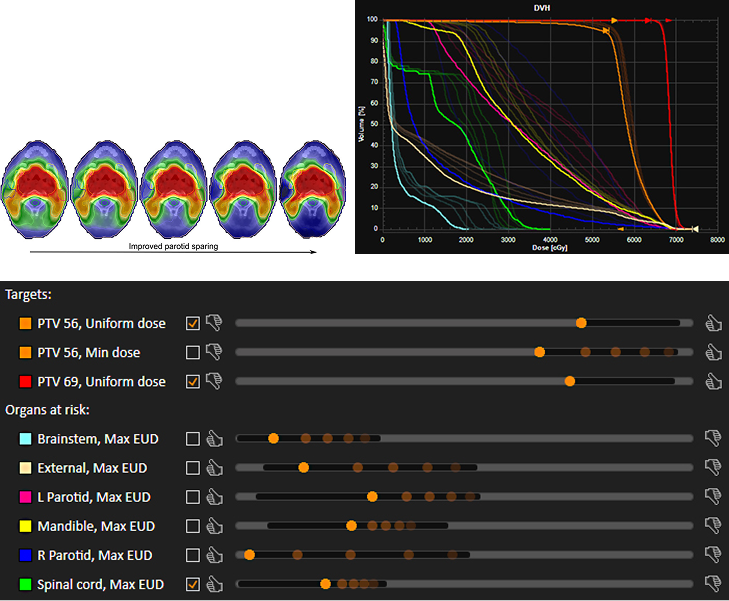
Figure 1. Continuous navigation toward improved sparing of the right parotid for a head-and-neck patient, shown as five snapshots in time.
(a): Transversal cuts of the navigated dose distribution.
(b): Overlaid DVHs with the final navigation state shown in bright colors.
(c): Overlaid navigation interface with the final slider positions shown in bright colors. The selected checkboxes indicate clamps, which makes parts of the slider ranges infeasible (grey regions).
CREATION OF A DELIVERABLE PLAN
The navigated dose distribution may be selected by the user for conversion to deliverable machine parameters. This conversion is performed by a dose mimicking optimization that minimizes discrepancies between the navigated dose and the deliverable plan. For fluence-based DMLC and all DMPO-based plan generation modes except for SMLC, the initial point of the optimization is calculated by interpolation of the Pareto plans according to the plan coefficients determined by the navigation. Such interpolation leads to a plan that accurately reproduces the navigated dose if the relationship between machine parameters and dose is linear (or close to linear).
The dose mimicking optimization seeks to recreate the navigated dose distribution on a voxel-per-voxel level if the Pareto plans are generated by DMPO. Otherwise, the navigated DVH is recreated. The objective function used during dose mimicking is composed of penalties defined across a user-specified set of regions of interest. Penalties associated with OARs are given unit weight while penalties associated with targets are given a weight equal to a user-defined target priority. The penalties have a one-sidedness behavior that is dependent on organ type: overdosage relative to the navigated dose is penalized for OARs and target voxels that have a cumulative volume less than 50 % according to the navigated DVH, whereas underdosage is penalized for other target voxels.
The dose mimicking optimization can be continued multiple times when necessary. It is also possible to continue the optimization in the plan optimization module by importing the dose mimicking optimization formulation from the MCO module.
CLINICAL RESULTS
The use of MCO has the potential to change clinical practice in several ways:
REDUCED PLANNING TIME AND IMPROVED QUALITY
Multiple studies have shown that MCO is more time-effective than standard inverse planning:
• Craft et al.[3] reported an average reduction in planning time from 135 to 12 minutes in planning for glioblastoma or pancreatic cancer.
• Wala et al.[4] reported an average planning time of 60 minutes per case when using MCO in planning for localized prostate cancer.
• Kamran et al.[5] reported a reduction of planning times from 193 to 107 minutes in planning for non-small cell lung cancer.
The plans generated by MCO were also in all three studies found to be of better quality than the plans generated by standard inverse planning according to blinded assessments by physicians.
LESS DEPENDENCY ON SKILL AND EXPERIENCE LEVEL OF THE PLANNER
• Kierkels et al.[6] showed that novice dosimetrists using MCO could create plans of comparable quality to plans created by experienced planners using standard inverse planning. Planning times were also shortened from 205 to 43 minutes on average when using MCO.
INCREASED PHYSICIAN INTERACTION AND IMPROVED DECISION MAKING
• Müller et al.[7] gave a proof of concept of MCO where the physician performed the navigation instead of the treatment planner. Workflows where treatment plans can be created in a single meeting between the physician and planner, as opposed to iterative plan adaptations by the planner based on feedback interspersed over long periods of time, has the potential to provide large time savings. Closer engagement of the physician in the treatment planning process may also influence clinical decision making, as indicated by the study by Müller et al. and others [8] where physicians prioritized clinical goals differently when being able to explore the possible treatment options through MCO.
REFERENCES
[1] Multi-criteria optimization for tomotherapy, RaySearch white paper, 2017.
[2] Bokrantz R and Forsgren A. An algorithm for approximating convex Pareto surfaces based on dual techniques. INFORMS J Comput 25(2): 377–393, 2013.
[3] Craft D, Hong T, Shih H, and Bortfeld T. Improved planning time and plan quality through multicriteria optimization for intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 82(1): e83–90, 2012.
[4] Wala J, Craft D, Paly J, Zietman A, and Efstathiou J. Maximizing dosimetric benefits of IMRT in the treatment of localized prostate cancer through multicriteria optimization planning. Med Dosim 38(3): 298–303, 2013.
[5] Kamran S, Mueller B, Paetzold P, Dunlap J, Niemierko A, Bortfeld T, Willers H, and Craft D. Multi-criteria optimization achieves superior normal tissue sparing in a planning study of intensity-modulated radiation therapy for RTOG 1308-eligible non-small cell lung cancer patients. Radiother Oncol 118(3): 515–20, 2016
[6] Kierkels R, Visser R, Bijl H, Langendijk J, van 't Veld A, Steenbakkers R, and Korevaar E. Multicriteria optimization enables less experienced planners to efficiently produce high quality treatment plans in head and neck cancer radiotherapy. Radiat Oncol 10: 87, 2015.
[7] Müller B, Shih H, Efstathiou J, Bortfeld T, and Craft D. Multicriteria plan optimization in the hands of physicians: a pilot study in prostate cancer and brain tumors. Radiat Oncol 12: 168, 2017.
[8] Hong T, Craft D, Carlsson F, and Bortfeld T. Multicriteria optimization in intensity-modulated radiation therapy treatment planning for locally advanced cancer of the pancreatic head. Int J Radiat Oncol Biol Phys 72(4):1208-14
* Subject to regulatory clearance in some markets.
For more information or to see a demo, contact sales@raysearchlabs.com