
MULTI-CRITERIA OPTIMIZATION FOR TOMOTHERAPY
Multi-criteria optimization (MCO) is a treatment planning approach that facilitates decision-making regarding conflicting dose requirements during treatment plan preparation. MCO makes it possible to modify a navigated dose in real time. This allows interactive exploration of a range of tradeoffs, such as how much the dose to an organ at risk must increase in order for a certain improvement in target coverage to be achieved. The navigated dose is formed as a weighted average of the doses for a set of pre-calculated treatment plans. TomoTherapy constitutes an ideal delivery technique for this type of plan averaging because the corresponding weighted average of the leaf-open times for the pre-calculated plan reproduces the navigated dose to a very precise approximation. Navigated doses are thus known to be achievable, so MCO for TomoTherapy constitutes a “what you see is what you get” feature.
PARETO PLAN GENERATION
MCO navigation relies on a set of pre-calculated Pareto plans that forms a representation of the (in general infinite) set of plans that are Pareto optimal with respect to the user-specified set of tradeoff objectives and constraints. RayStation’s full set of optimization capabilities for TomoTherapy is available during the generation of the Pareto plans, including dynamic jaw tracking of the target volume, delivery time constraints and the possibility to specify “protect” volumes that prohibit irradiation on the entrance side, or both sides, of the target. Pareto plans can be optimized in regard to:
• Dose calculated using a fast but approximate singular-value decomposition (SVD) dose algorithm.
• A mixed dose calculated as SVD dose increments that are added to a more accurate background dose. This background dose is calculated using a collapsed cone (CC) dose algorithm.
A final CC dose calculation can also be performed.
DELIVERABLE PLAN CREATION
On the user’s command, a navigated dose is translated into a deliverable treatment plan by an optimization that minimizes the difference between the navigated dose and the dose of the deliverable plan (the deliverable dose). This optimization is initiated from a plan that is constructed as follows:
• The leaf-open times for the Pareto plans are calculated based on their projection time and set of leaf-open ratios.
• A weighted average of the leaf-open times is calculated, with the weight coefficients in the average determined according to each Pareto plan’s contribution to the navigated dose.
• The averaged leaf-open times are converted to a projection time and set of leaf-open ratios that are feasible with respect to the machine constraints.
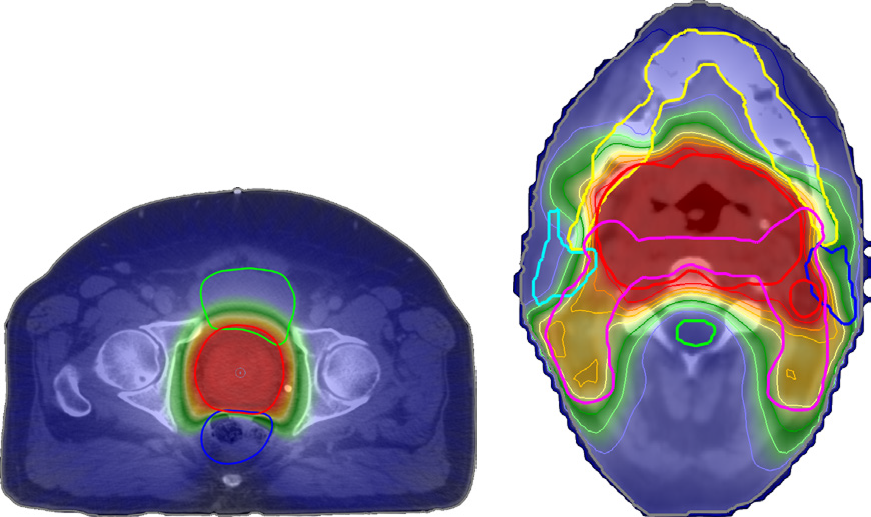
A deliverable plan created by this form of plan averaging and dose difference minimization accurately recreates the navigated dose. This is illustrated in Figure 1, which shows representative results for the creation of a deliverable plan for a prostate and a head and neck patient. The figure shows that no voxel had a dose error greater than 1% of the prescription and that the small existing errors were primarily located outside of the high-dose region. The navigated doses used as input are shown in Figure 2. Both patients were planned for treatment with a prescription of 70 Gy over 30 fractions. All depicted doses were calculated using the CC dose algorithm.
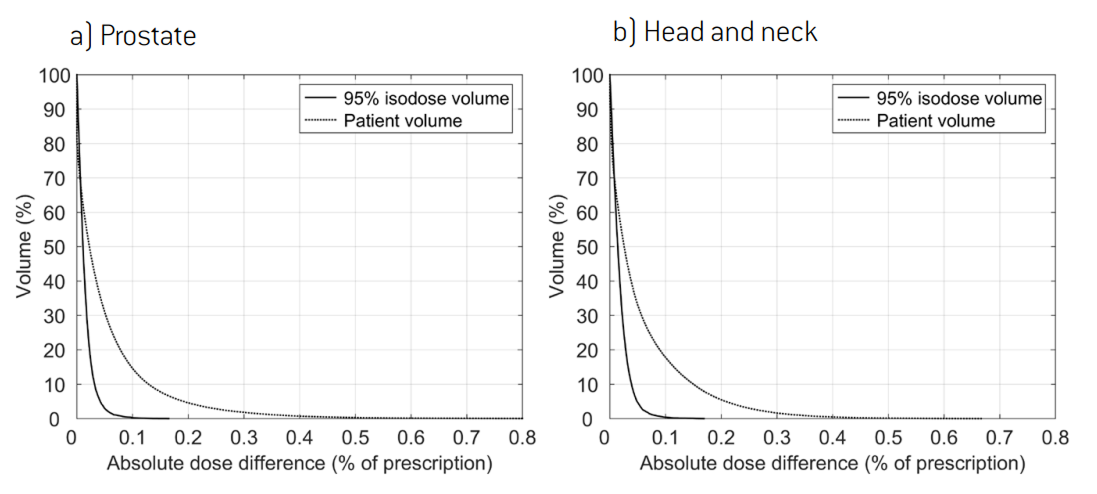
1 A treatment plan is Pareto optimal if it is feasible with respect to the constraints and no objective can be improved without a sacrifice for at least one other objective.
DELIVERY EFFICIENCY TRADEOFFS
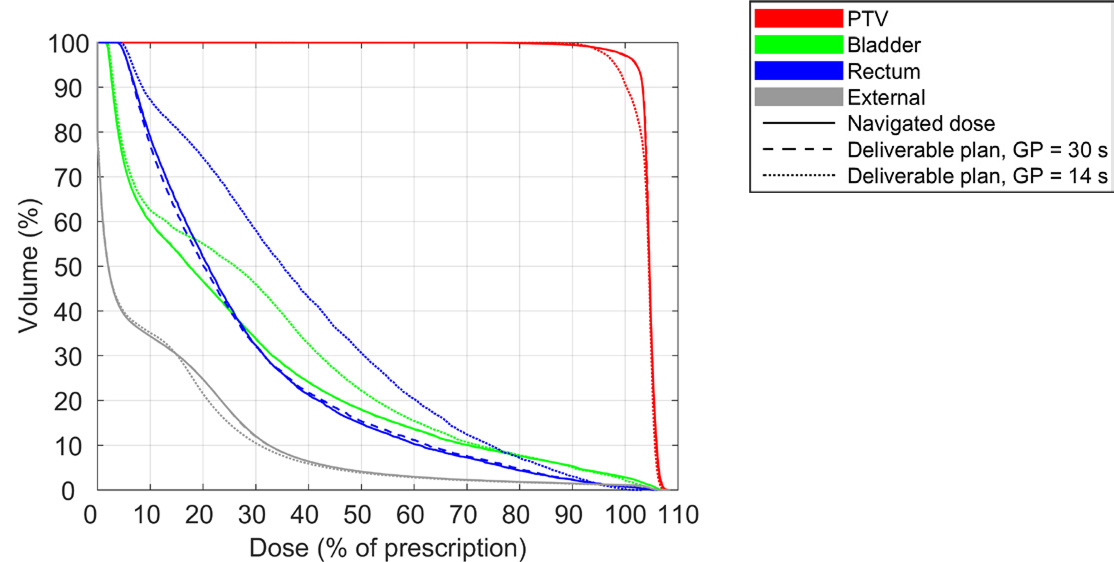
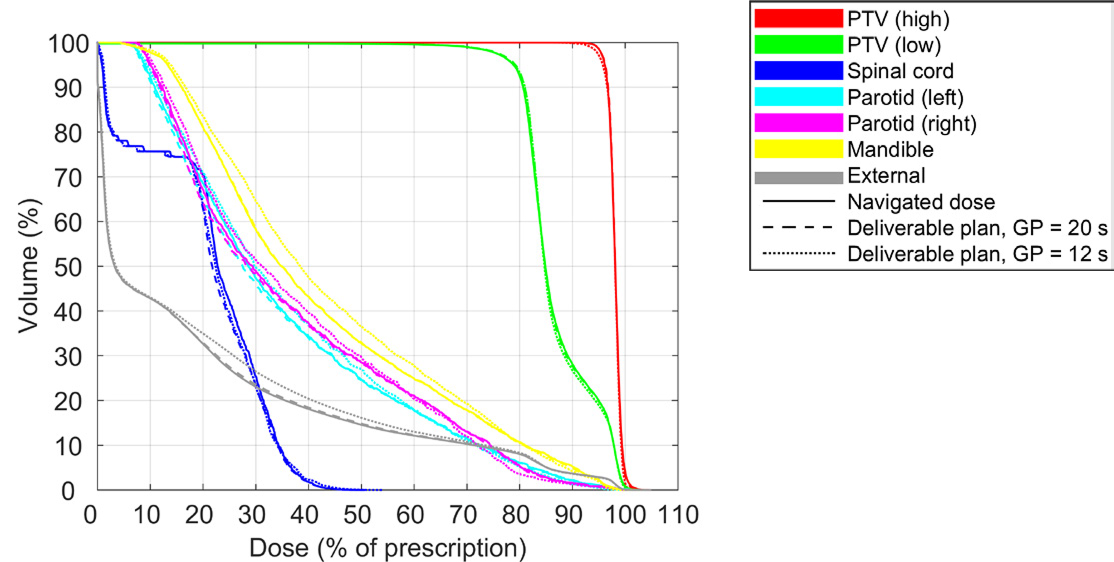
It can be difficult to define constraints on delivery time prior to the generation of Pareto plans when the impact of such constraints is unknown. One approach to find a suitable bound on the delivery time is to generate Pareto plans with a high or unlimited maximum delivery time. The upper limit on the delivery time can then be gradually decreased during the creation of a deliverable plan, until the difference between the navigated dose and deliverable dose becomes too large. This approach is illustrated in Figure 3 for the prostate patient and in Figure 4 for the head and neck patient, which depict dose-volume histograms (DVHs) for the navigated dose, and for the deliverable dose at selected maximum gantry periods. The figures show that the gantry period could be reduced to 30 s for the prostate patient and 20 s for the head and neck patient with negligible impact on the DVH. A further reduction of the delivery time to 14 s and 12 s, respectively, led to overdosage of the organs at risk and an underdosage of the target(s) compared to the navigated doses. A clinician might opt for a treatment delivery time such that the difference between the navigated dose and deliverable dose is non-negligible but clinically insignificant.
CONCLUSION
The inclusion of support for both TomoHelicalTM and TomoDirectTM treatment techniques in RayStation permits users to benefit from MCO, which allows continuous and immediate exploration of tradeoffs between planning objectives. The fact that navigated doses can be reconstructed by a deliverable plan to sub-percent accuracy makes TomoTherapy an ideal treatment technique for MCO. An appropriate bound on the treatment delivery time can be identified through investigation of the tradeoff between delivery efficiency and plan quality during the creation of a deliverable plan.
2 RayStation supports three different forms of delivery time constraints: maximum delivery time, maximum gantry period and maximum delivery time factor. A delivery time factor of x means that the delivery time cannot exceed x∙t, where t is the delivery time when the leaf-open times are uniform and scaled such that the average target dose equals an estimate of the prescription (based on the current set of optimization functions).
For more information or to see a demo, contact sales@raysearchlabs.com